|
HCU Clinical Trials |
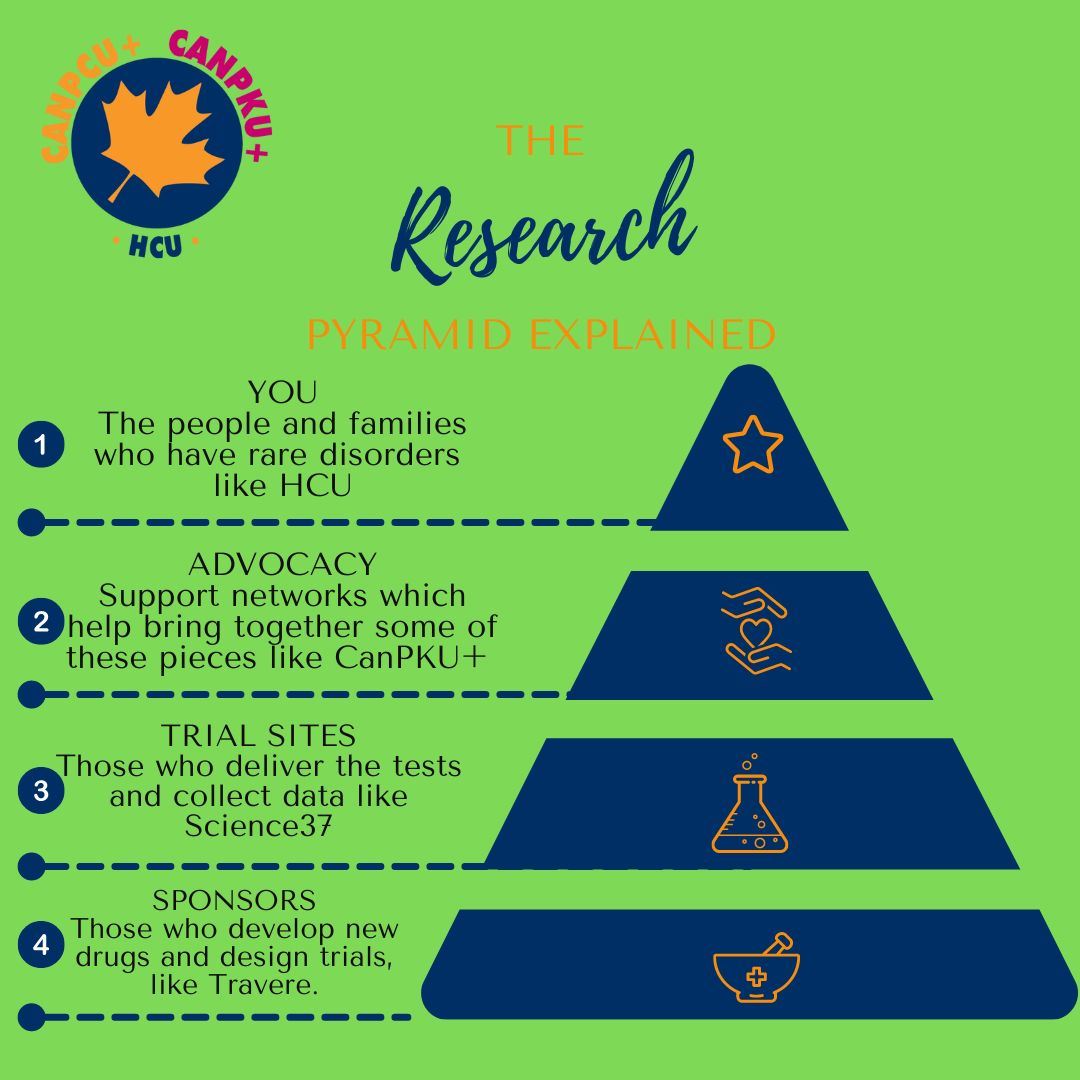
CanPKU+ is very active in helping in any we can in order to advancing the HCU Tool box by working with clinical trials in order to get more in the tool box. |
More information for trials that have requested our assistance as they are accepting Canadians:
THIS STUDY IS CURRENTLY NO LONGER TAKING APPLICANTS
Science 37 along with Travere are doing a natural history study. This study does not change the person with Classic HCU's day to day life and is not introducing any intervention or therapy. It's purpose is to gather information on the lives of those with Classic HCU. The clinical trials website does not yet reflect the Canadian site. The Canadian site will be virtual with visiting nurses when bloodwork is required. (They will come to you). This is currently being offered for Canadians with Classical HCU in BC, AB and ON. Next up will be QC.
Inclusion Criteria:
- Patients who are clinically diagnosed with homocystinuria
- Male/female patients aged 1 to 65 years
- Patients who consented and/or assented
- Patients who are willing and able to comply with all study-related procedures.
Exclusion Criteria:
- Medically significant postnatal complications or congenital anomalies that are not associated with homocystinuria
- Received any experimental therapy for homocystinuria during the 6 months prior to enrollment or expected to receive any such therapy during duration of the study
More information can be found at
https://clinicaltrials.gov/ct2/show/record/NCT02998710recrs=ab&cond=Homocystinuria&draw=2&rank=2
What is a Natural History Study? Find out more with Insightful Moment's ROADMAP. Qu'est-ce qu'une étude d'histoire naturelle? Découvrez-en plus avec la FEUILLE DE ROUTE d'Insightful Moment.
|
Insightful Moments has produced a ROADMAP for finding a clinical trial. A site listing all clinical trials over a wide range of countries: https://clinicaltrials.gov/ PTC Therapeutics, one of CanPKU+ Sponsors offers clinical trials via their site: https://clinicaltrials.ptcbio.com/en/ Synlogic, one of CanPKU+ sponsors offers clinical trials via this site: Index (pkuresearchstudy.com) Clinical Trials Ontario Clinical Trials Ontario| Clinical Trials Ontario (ctontario.ca) for info on trials in Ontario |
|
|
CommuniKIDS team was thrilled to share the finalized trial results template and “tip sheet” They hope that the template which was developed together will be used widely and will be helpful to youth and their families after participating in a clinical trial. This was officially launched on the CTO website this Friday, May 20th, 2022 on International Clinical Trials Day, where trialists will be able to download the template for their own use to communicate trial results back to youth and their families. They expect it to be available in french by the summer of 2022. They acknowledge and thank those who provided input including 5 youth advisors, Allison, Chloe, Jessica, Rhys, and Samarah, and 8 parent advisors, including Katrina Hill, Karen Ladner Haas, Lynn Mendonza, Ryan LaPointe, Tanya Chute Nagy, and Yan Défossés. This work was supported by the CHILD-BRIGHT KT Innovation Knowledge Incubator grant. |